KILR® Cytotoxicity Bioassays
Robust, Ready-to-Use Bioassays to Measure Direct Cell Death
The KILR cytotoxicity assay platform provides a robust and sensitive solution to measure the killing of antigen-expressing target cells in co-culture with effector cells. The platform allows for the quantification of direct cell death through multiple mechanism of actions (MOAs), including ADCC (antibody-dependent cell-mediated cytotoxicity), ADCP (antibody-dependent cellular phagocytosis), CDC (complement-dependent cytotoxicity, and T-cell redirection.
Eurofins DiscoverX®’s KILR bioassays are plate-based, homogeneous ready-to-use (RTU) cell-based assay kits that include all necessary reagents to run the assay. These physiologically relevant, robust bioassays are fit-for-purpose for use in screening and characterization applications to potency testing in quality control (QC) lot-release programs. The KILR RTU bioassays demonstrate extensive lot-to-lot consistency in assay performance and help ensure long-term assay reproducibility with high signal-to-background ratios, key factors in potency lot-release testing programs. The KILR platform provides the flexibility to use your effector cells of choice. Additional custom assay development capabilities are available to generate optimized bioassays with your target cell model of interest and for relevant data generation.
Product Highlights
- Multiple Applications – Analyze ADCC and ADCP in screening and characterization applications to potency testing in QC lot-release programs
- Comparable Performance – Excellent concordance in assay performance between continuous culture and RTU bioassay formats
- Simple Protocol, Fast Results – Easy-to-run, rapid homogeneous protocol amenable to implementation in multiple labs and high-throughput format for increased efficiency
- Reproducible Results – Ensure long-term assay reproducibility with high signal-to-background ratios
KILR Bioassay Kits
| Bioassay Kit Name | Cancer Cell Type | Target | Assay Measures | Drug Name | Bioassay Kits & Configuration |
|---|---|---|---|---|---|
| KILR® NCI-N87 ADCC Bioassay Kit | HER2 | Antibody-Dependent Cellular-Cytotoxicity | 97-1004Y021-00173 (2-plate) 97-1004Y021-00174 (10-plate) | ||
| KILR Raji ADCC Bioassay Kit | Burkitt’s lymphoma | CD19, CD20, CD38 | Antibody-Dependent Cellular-Cytotoxicity | Rituximab | 97-1012Y026-00169 (2-plate) 97-1012Y026-00170 (10-plate) |
| KILR Daudi ADCC Bioassay Kit | Burkitt’s lymphoma | CD19; CD20; CD38 | Antibody-Dependent Cellular-Cytotoxicity | Rituximab | 97-1009Y025-00171 (2-plate) 97-1009Y025-00172 (10-plate) |
| KILR Raji ADCP Bioassay Kit | Burkitt’s lymphoma | CD19; CD20; CD38 | Antibody-Dependent Cellular-Phagocytosis | Rituximab | 97-1012Y026-00179 (2-plate) 97-1012Y026-00180 (10-plate) |
| KILR Daudi ADCP Bioassay Kit | Burkitt’s lymphoma | CD19; CD20; CD38 | Antibody-Dependent Cellular-Phagocytosis | Rituximab | 97-1009Y025-00177 (2-plate) 97-1009Y025-00178 (10-plate) |
Request qualification data set for KILR® Raji ADCC Bioassay Kit using rituximab.
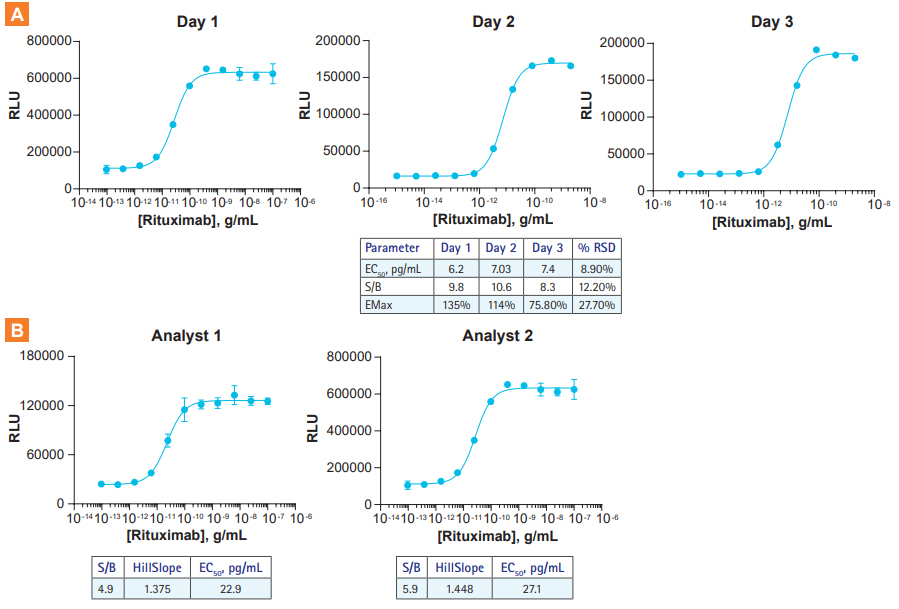
KILR ADCC bioassays deliver high repeatability and reproducibility. A. A dose response of the anti-CD20 antibody Rituximab was evaluated in the respective target cells by a single analyst on three different days using KILR Raji bioassay target cells and KILR CD16 Effector Cells. The results demonstrate the low variability in EC50, S/B (signal-to-background), and EMAX values over the three days indicating the high repeatability of the ADCC assay. Comparable data was obtained with the KILR Daudi model (data not shown). B. Functional response analysis of KILR ADCC bioassays by two analysts over two days shows excellent concordance in curve shape, S/B, and EC50.
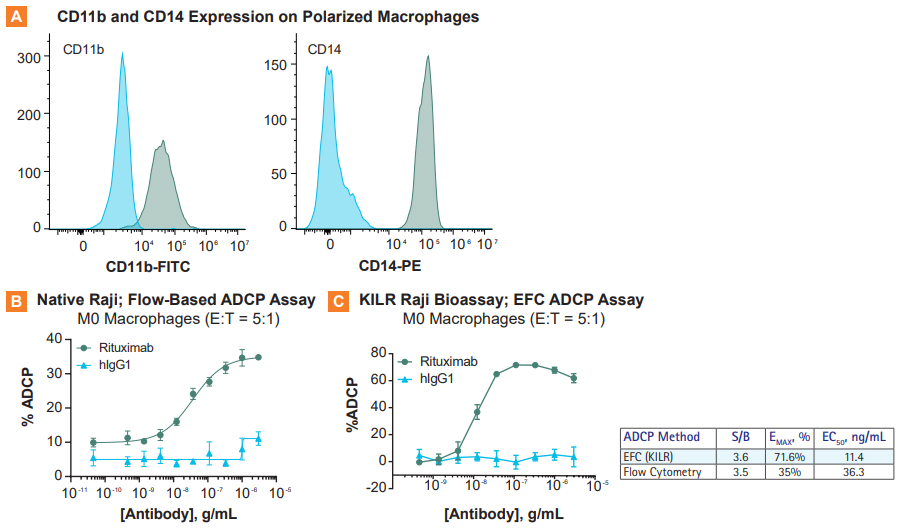
Comparison of rituximab-mediated ADCP in two different assay formats – flow cytometry vs the KILR enzyme fragment complementation (EFC) format. Monocytes from the same healthy donor were isolated and differentiated to M0 macrophages prior to use in each assay. A. Expression of CD11b and CD14 was assessed on the polarized macrophages by flow cytometry at the time of harvest to confirm the differentiation state. B. Flow-based and C. KILR EFC-based assay results normalized to vehicle-treated cells to calculate % ADCP. Overall, a higher percentage of phagocytosis was observed when using the KILR assay, indicating a more effective ADCP assay format. E:T = Effector cells (macrophage):target.

A well-structured process for bioassay development yields assays that are robust and reproducible. The process begins with selecting the assay that most appropriately reflects the MOA of the drug, followed by optimization of assay parameters. Optimization through qualification steps includes refining conditions with the originator drug or reference molecule to establish robustness, precision, accuracy, and linearity of assay.
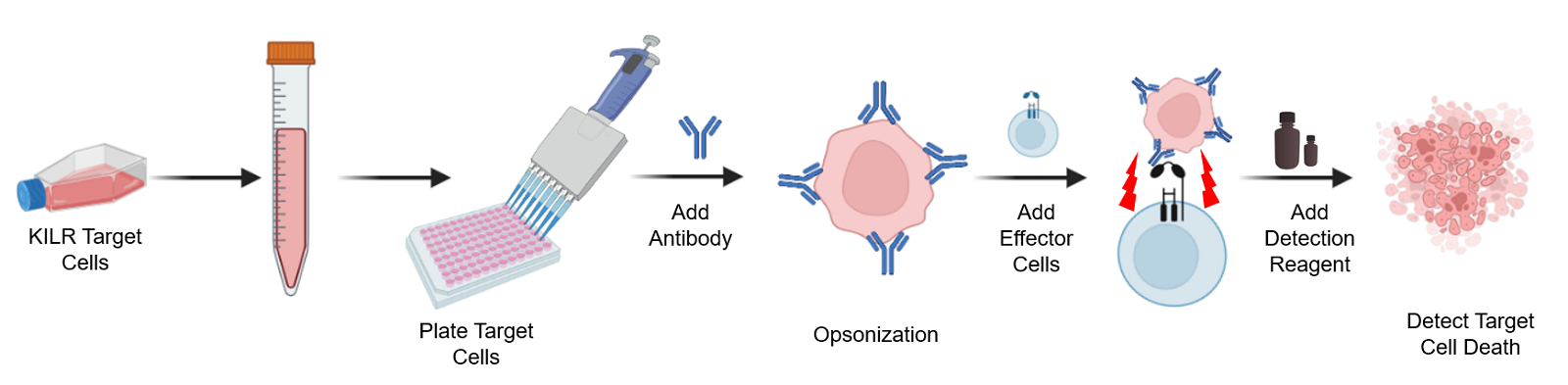
The KILR ADCC assay workflow. KILR target cells of interest are engineered to stably express a housekeeping protein that is tagged with a reporter fragment (KILR reporter protein). These cells are plated, followed by the addition of the test antibody to the target cells. Following opsonization, effector cells (e.g. KILR CD16 Effector Cells) are added to the wells containing the target cells. Effector-mediated killing releases the KILR reporter protein into the media that is detected by the addition of detection reagents and results in a chemiluminescent signal that can be read on any standard benchtop luminometer.
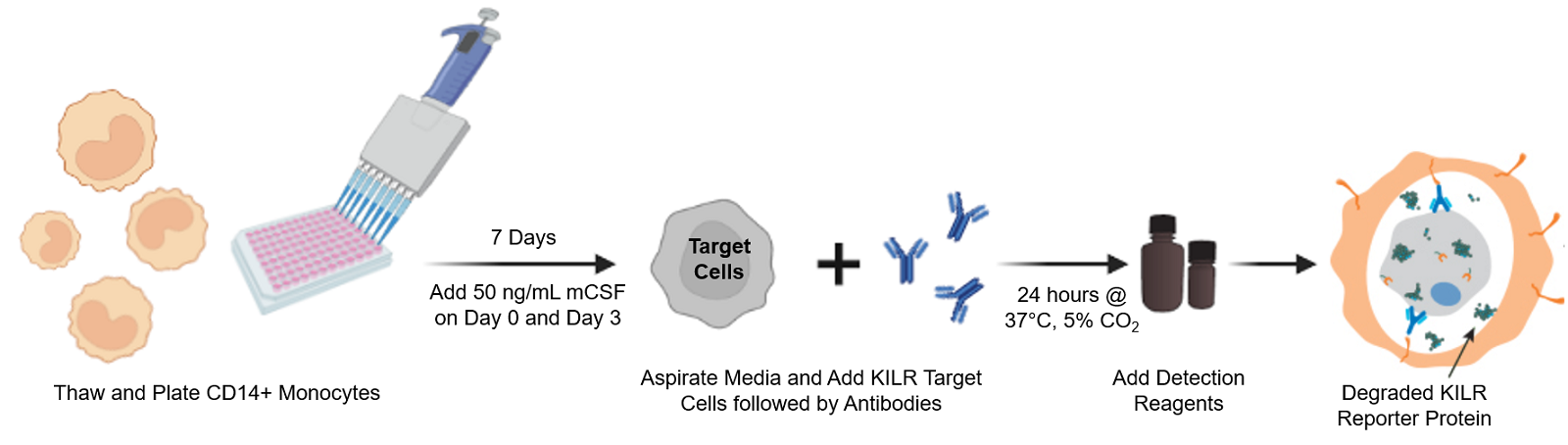
The KILR ADCP assay workflow. CD14+ Monocytes are thawed and plated with the addition of 50 ng/mL of mCSF. Following 7 days of incubation, KILR target cells of interest, expressing the KILR reporter protein and a test antibody, are added to the plate wells containing the monocytes, now differentiated into macrophages. The macrophages phagocytose the opsonized KILR target cells leading to the degradation of the KILR reporter protein. The addition of detection reagent causes cell lysis. Complementation of two β-gal fragments with the addition of substrate results in a dose-dependent decrease in chemiluminescent signal with increasing concentrations of the test antibody. Note a shorter protocol is available and included within the ADCP application note.
The KILR platform is based on the industry-validated Enzyme-Fragment Complementation (EFC) technology. EFC is based on two recombinant β-galactosidase (β-gal) enzyme fragments that act as an enzyme acceptor (EA) and an enzyme donor (ED). Separately, the fragments are inactive, but when combined, they form an active β-gal enzyme that hydrolyzes its substrate to produce a chemiluminescence signal.
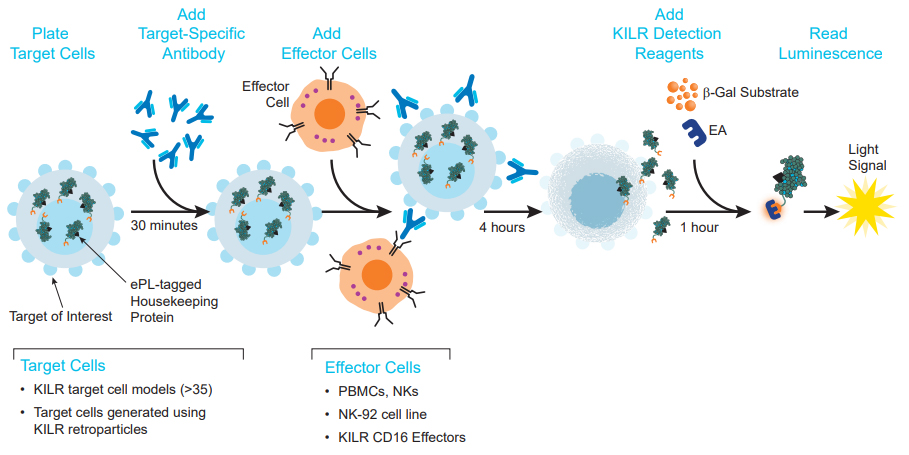
The KILR ADCC assay principle. Target cells expressing the relevant antigen are engineered to stably express a housekeeping protein tagged with a reporter β-gal ED fragment called enhanced ProLabel® (ePL). The β-gal enzyme is inactive when the reporter fragment (also called the KILR reporter protein) is not paired with its larger β-gal EA fragment. On incubation of KILR target cells with a test antibody and appropriate effector cells (e.g. KILR CD16 Effector Cells), effector cell-mediated killing releases the tagged protein into the media. The presence of both EA and ED fragments leads to the formation of the active β-gal enzyme that hydrolyzes the substrate to give a chemiluminescent output detected on any benchtop luminometer.
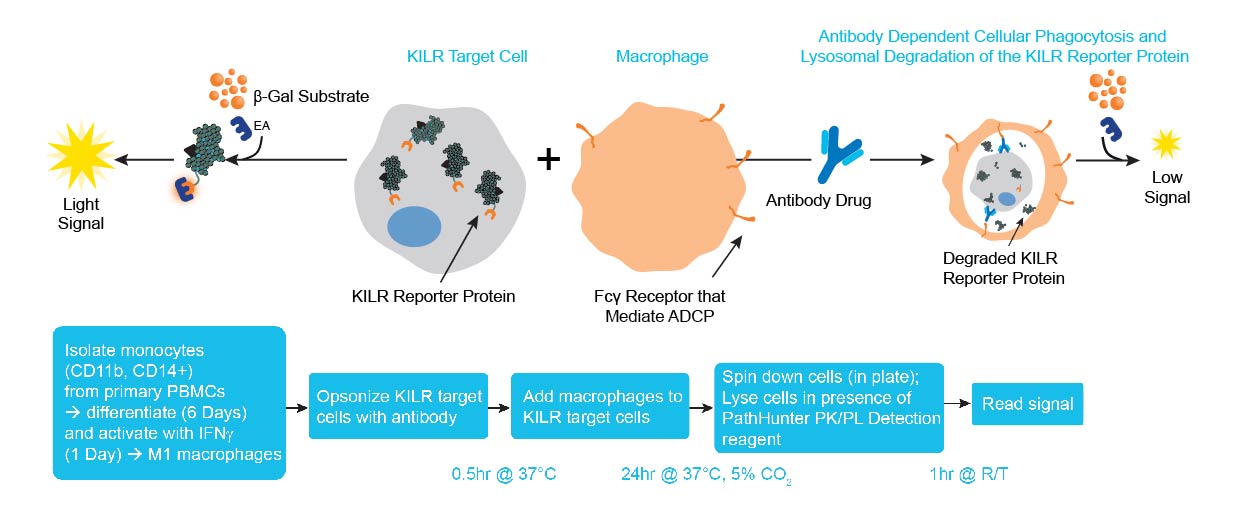
The KILR ADCP assay principle. ADCP measures antibody-dependent phagocytosis of target cells, typically mediated by primary human macrophages. KILR target cells, containing the KILR reporter protein (including the β-gal ED fragment also called ePL), undergo phagocytosis by effector cells (e.g. macrophages differentiated from monocytes) leading to lysosomal degradation of the KILR reporter protein. In this assay, the KILR target cells are opsonized with the antibody of interest, then co-cultured with the effector cells. Macrophages perform phagocytosis of the target cells. All non-phagocytosed cells are lysed and the detection reagent containing the complementing β-gal EA fragment is added to the lysed cells. Complementation of ED and EA with the addition of substrate results in the detection of the non-phagocytosed KILR protein present. A high chemiluminescent signal indicates minimum killing, while a low signal correlates to higher phagocytosis or increased ADCP activity. Note: other immune effector cells can also be used for the ADCP assay.
Screen and Rank Order Antibodies
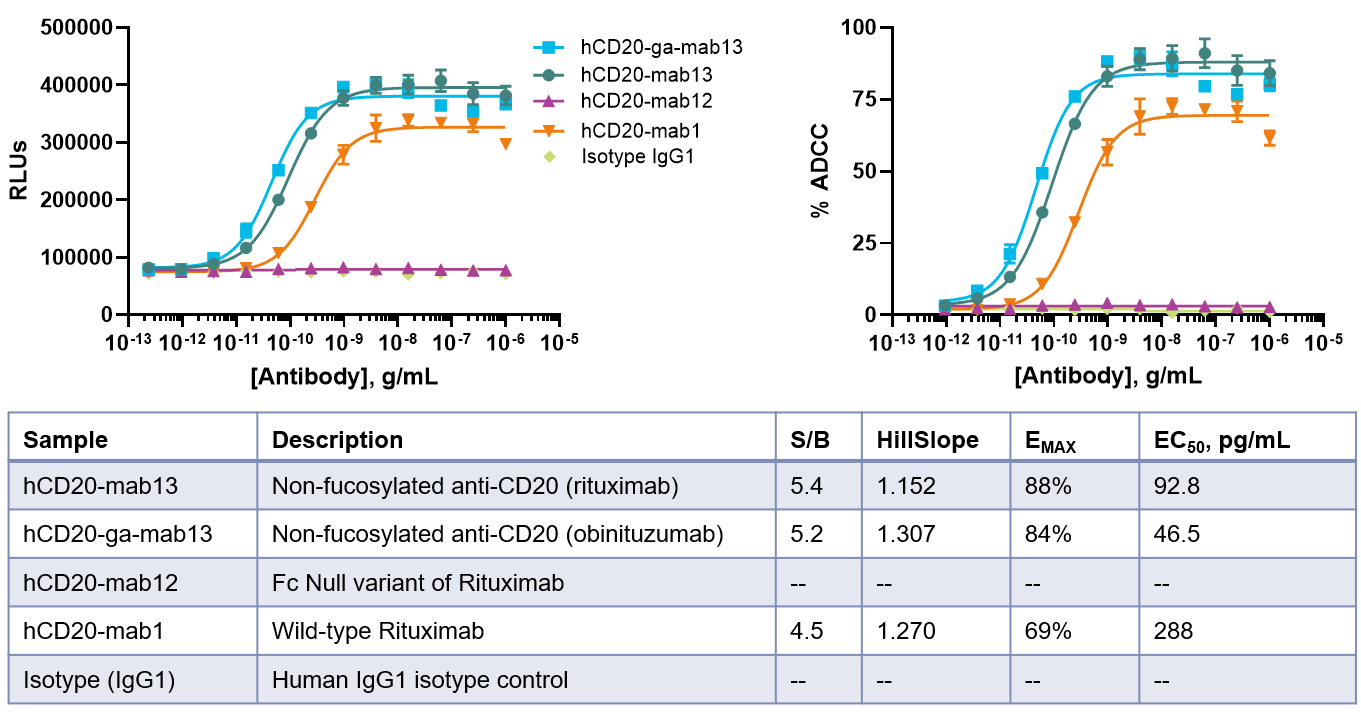
Screen and rank order antibodies with KILR Bioassays. Quantify the differences in target cell killing due to the change in both Fab and Fc regions of antibodies, thus allowing rank ordering of therapeutic antibodies. Results show variants of the CD20 antibody rituximab with wild-type CD20 Rituximab (hCD20-mab1) being the least potent.
Perform Potency Assays
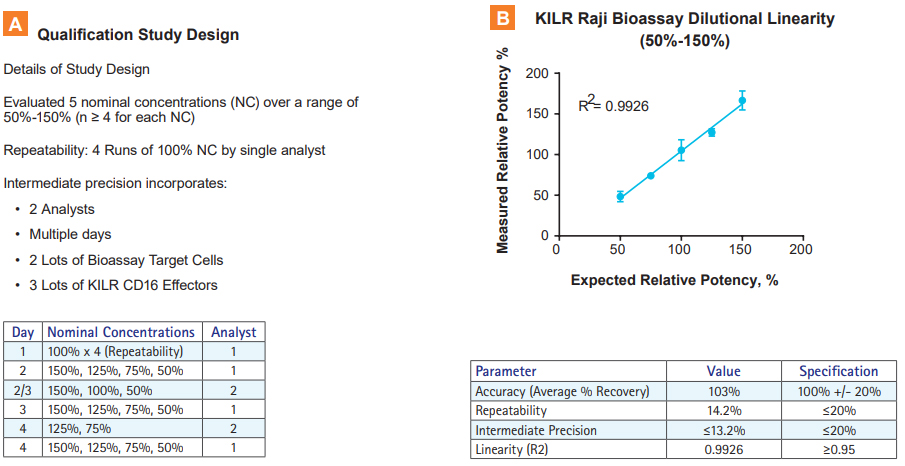
Qualification data for KILR Raji Bioassay using rituximab demonstrating the assays ability to determine relative potencies. A. Study design for qualification of KILR Raji ADCC Bioassay. Five nominal concentrations of rituximab were evaluated over a range of 50% to 150%. Repeatability (4 runs) was assessed at the 100% nominal concentration by a single analyst. Intermediate precision incorporated several sources of variability, including multiple analysts, multiple days, 2 lots of KILR Raji Bioassay target cells, and 3 different lot of KILR CD16 Effectors. B. Results obtained from the qualification study indicate very good accuracy, high repeatability, intermediate precision, and dilutional linearity for KILR Raji Bioassay.
Evaluate ADCP Activity of Therapeutics with Fc Regions

The KILR ADCP Bioassay Kit can be used to evaluate the ADCP activity of different antibody therapeutics that utilize IgG1 or IgG4(S228P) Fc domains. IgGx affinity for Fc receptors (impact on immune-mediated killing) via ADCP application is in the following order: IgG1 > IgG3 > IgG2 > IgG4. These data specifically show that anti-PD1 (or anti-CD20) therapeutics that utilize an IgG4(S228P) Fc (e.g., Pembrolizumab and Nivolumab) mediate ADCP as effectively as IgG1-formatted antibodies. Therefore, biosimilar developers of anti-PD-1 (or CD20) therapeutics should consider testing the ADCP activity of their drugs during the development and characterization of their biosimilars.
The development of antibody-based therapeutics to target the killing of tumor cells has revolutionized the space of immuno-oncology and the…
Read MoreWith the increasing industry focus on antibody drugs, there is an ever-greater need for functional bioassays that interrogate the therapeutic…
Read MoreGaurav Agrawal, Ph.D.Scientific Development Manager
Read MoreDiscoverX CASSS 2017 Presentation Presented by Dr. Jane Lamerdin, Director R&D, DiscoverX May 9, 2017
Read MoreJane E. Lamerdin, Ph.D. Vice President, Research & Development Eurofins DiscoverX
Read MoreCell-based assays play an important role in determination of mechanism of action (MOA) for therapeutic antibodies, particularly when evaluating their…
Read MoreThe clinical success of an ever-increasing array of biologics has led to the development of a wide spectrum of immunomodulatory…
Read MoreComprehensive Eurofins DiscoverX product list to enable your drug discovery and development programs focused on checkpoint receptors, cytokine receptors/interleukins, cytotoxicity,…
Read MoreAccelerate your biologics development from discovery to QC lot release with cell-based assays. Comprehensive list of bioassay kits, cell lines,…
Read MoreThe webinar focusses on measuring antibody-mediated cell death using DiscoverX’s KILR Bioassays, and how they can be used to measure…
Watch NowTherapeutic regulators require assay data on the impact of Fc effector-mediated function of antibodies during their development as therapeutics. The…
Read MoreLearn how KILR® cytotoxicity assays provide a simple, non-radioactive and dye free method to specifically measure target cell death in…
Watch NowTherapeutic antibodies that can elicit different applications of cell-mediated cytotoxicity, such as Antibody-Dependent Cell-mediated Cytotoxicity (ADCC) and many other effector…
Read MoreRobust & Reproducible Cell-Based Assays for Potency Testing
Read MoreRobust, Ready-to-Use ADCC & ADCP Bioassays to Accelerate Your Drug Development Program The KILR® cytotoxicity assay platform developed by Eurofins…
Read MoreProducts & Custom Development Capabilities.
Read More
Simple, non-radioactive, & dye-free cytotoxicity assays to specifically measure target cell death
Read More
Single donor-derived human primary cells to drive robust & reproducible ADCC & T-cell redirection
Read More
Custom cell lines, kits, assays, & protein development capabilities optimized to fit your requirements
Read More
