| Biological Information | |
|---|---|
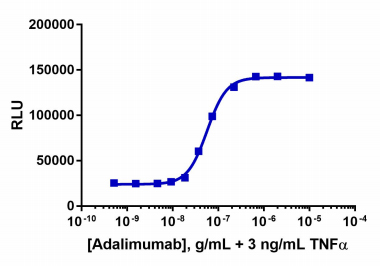
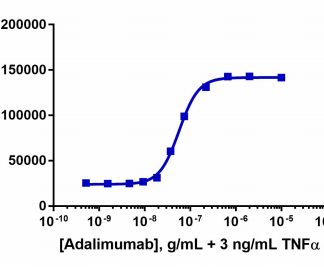
| Background Information: | The PathHunter® Adalimumab Bioassay Kit provides an easy-to-use cell based assay to measure drug potency and detect neutralizing antibodies. This bioassay is designed to measure IkB degradation as a result of TNF alpha-mediated activation of the NF-kB signaling pathway. The included cells overexpress IκB tagged with PL. Addition of the detection reagent containing EA, forces complementation of the two enzyme fragments. The resulting active enzyme hydrolyzes the substrate to generate a chemiluminescent signal which is proportional to the degree of IκB stabilization. This assay has been optimized and qualified with Humira (not supplied in the bioassay kit). Humira is a registered trademark of AbbVie, Inc. The bioassay kit contains all the materials needed to run the assay, including cryopreserved cells, cell plating reagent, control agonist, detection reagents, and assay plates. |
| Target Class: | Cytokine |
| Family: | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| Accession Number: | NM_020529 |
| Target Name: | TNFa |
| Target Aliases: | IKBA, MAD-3, NFKBI, EDAID2, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| Target Species: | Human |
| Cell Background: | A549 |
| Usage | |
| Product Type: | Bioassay Kits |
| Application: | Potency; Commercial Release & Stability |
| Kit Components: | 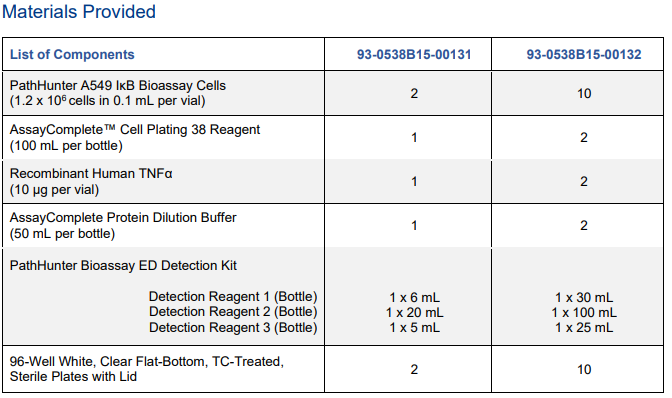 |
| Storage Conditions: | Store in vapor phase of liquid nitrogen. |
| Usage Disclaimer: | These products may be covered by issued US and/or foreign patents, patent application and subject to Limited Use Label License. Please visit discoverx.com/license for a list of products that are governed by limited use label license terms and relevant patent and trademark information. |
| Assay Information | |
| Assay Type: | Functional |
| Assay Measures: | SH2 Recruitment |
| Detection Method: | Chemiluminescence |
| Bioassay Data | |
| Qualified With: | Humira® |
| Trademark Statement: | Humira® is a registered trademark of AbbVie, Inc. |
| Clinical Relevance | |
| Therapeutic Area: | Inflammation/Allergy |
| Additional Information | |
| Brand: | PathHunter® |
PathHunter® Adalimumab Bioassay Kit
The PathHunter® Adalimumab Bioassay Kit provides an easy-to-use cell based assay to measure drug potency and detect neutralizing antibodies. This bioassay is designed to measure IkB degradation as a result of TNF alpha-mediated activation of the NF-kB signaling pathway.
View bioassay qualification data.
Learn about analytical cell banks for bioassays as part of the critical reagents management program.
User Manuals & Protocols
70-412 PathHunter Adalimumab Bioassay Kit User Manual REV0
View DocumentDatasheets
93-0538B15x Datasheet
View Document