| Biological Information | |
|---|---|
| Background Information: | The PathHunter® Bevacizumab Bioassay HS Kit provides an easy-to-use cell based assay to measure drug potency and detect neutralizing antibodies. This bioassay detects VEGF-A induced Homodimeriziaton of the kinase insert domain (KDR), also known as VEGFR2 receptor. The included cells overexpress ProLink-tagged KDR/KDR and EA-tagged. Activation of KDR/KDR stimulates the recruitment of and produces EFC signal. This assay has been optimized and qualified with Avastin® (not supplied in the bioassay kit). Avastin® is a registered trademark of Genentech, Inc.. Bioassay kits are a convenient, ready-to-use format which contains all materials needed to run the assay, including single-use vials of cryopreserved cells, cell plating media, control agonist, detection reagent, and assay plates. The HS kit has been optimized to deliver reproducible results with lower variability. |
| Target Class: | Kinase |
| Family: | Receptor Typosine Kinase (RTK): Growth Factor Receptor |
| Accession Number: | NM_002253.2 |
| Target Name: | KDR/KDR |
| Target Aliases: | Kinase Insert Domain Receptor, FLK1, CD309, VEGFR, VEGFR2 |
| Target Species: | Human |
| Cell Background: | HEK293 |
| Usage | |
| Product Type: | Bioassay Kits |
| Application: | Potency; Commercial Release & Stability |
| Kit Components: | 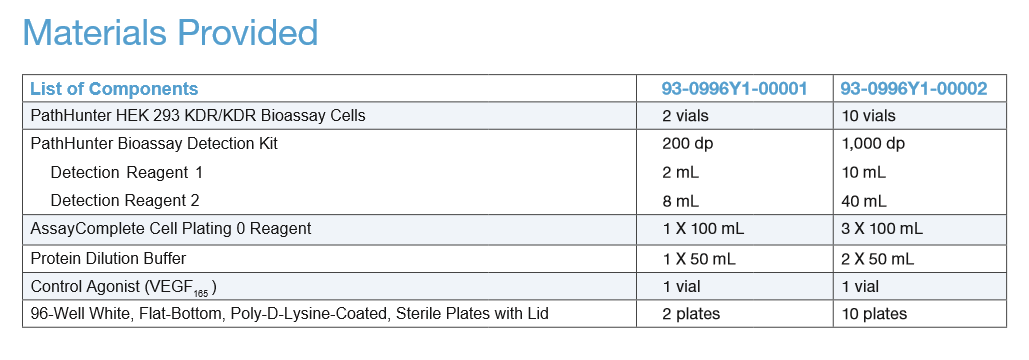 |
| Storage Conditions: | Store in vapor phase of liquid nitrogen. |
| Usage Disclaimer: | These products may be covered by issued US and/or foreign patents, patent application and subject to Limited Use Label License. Please visit discoverx.com/license for a list of products that are governed by limited use label license terms and relevant patent and trademark information. |
| Assay Information | |
| Assay Type: | Functional |
| Assay Measures: | Dimerization |
| Detection Method: | Chemiluminescence |
| Bioassay Data | |
| Qualified With: | Avastin® |
| Trademark Statement: | Avastin® is a registered trademark of Genentech, Inc. |
| Clinical Relevance | |
| Therapeutic Area: | Oncology/Immuno-Oncology |
| Additional Information | |
| Brand: | PathHunter® |
PathHunter® Bevacizumab Bioassay HS Kit

The PathHunter® Bevacizumab Bioassay HS Kit provides an easy-to-use cell based assay to measure drug potency and detect neutralizing antibodies. This bioassay detects VEGF-A induced homodimeriziaton of the kinase insert domain (KDR), also known as VEGFR2 receptor.
View bioassay qualification data.
User Manuals & Protocols
70-435 PathHunter Bevacizumab Bioassay HS Kit REV0 User Manual
View DocumentDatasheets
93-0996Y1x Datasheet
View Document
